Chicago, USA: April 25, 2025 -- Altis Labs, Inc. (“Altis”) is announcing results presented at the American Association for Cancer Research (AACR) 2025 in Chicago: Baseline IPRO-α outperformed RECIST 1.1 Sum of Longest Diameters in stratifying overall survival in AstraZeneca’s Ph3 aNSCLC trial, MYSTIC.
In this evaluation, Altis’ AI pipeline was applied to baseline imaging collected in the MYSTIC trial. AstraZeneca compared the prognostic association of manual RECIST 1.1 versus AI-derived quantifications: IPRO-α and Volumetric Tumor Burden (VTB).
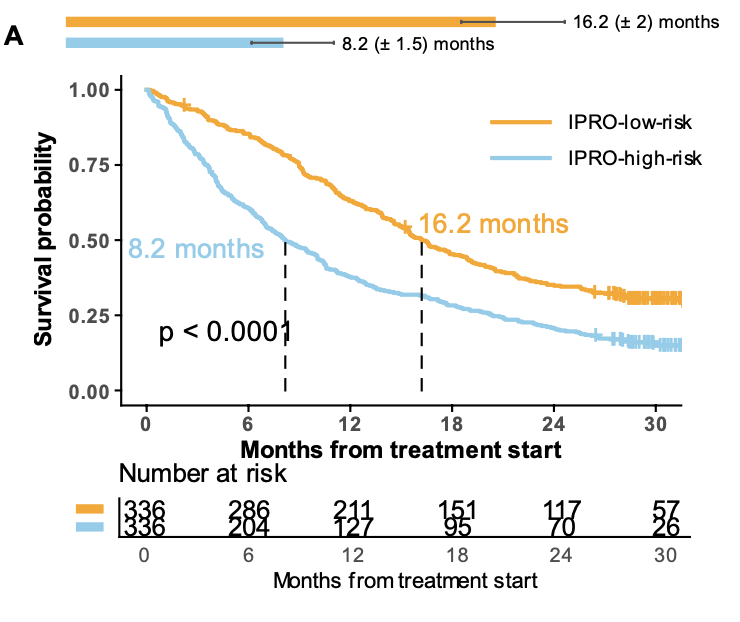
-IPRO-α had a greater association with OS (HR=0.57, 95% CI: 0.48-0.68) than SLD (HR=0.76, 95% CI: 0.64, 0.90),
-Lung Volumetric Tumor Burden (Lung VTB), an AI-based measure of disease burden, showed similar prognostic association (HR=0.68, 95% CI: 0.57-0.81) as SLD.
These results demonstrate that AI-derived imaging biomarkers like IPRO not only match but may exceed conventional measurements in quantifying disease burden. These tools could enable more nuanced patient stratification and unlock more confident measures of treatment effects—without manual measurements.
Access the abstract here.
About Altis Labs
Altis Labs is the computational imaging company accelerating clinical trials with AI.
Altis has created the industry's largest real-world Imaging, Clinical, and Outcomes database – comprising over 210 million cancer patient images linked to associated demographic, diagnostic, molecular, treatment, and survival information. Deep learning models trained on rwICO can generate profound insights beyond tumor measurements that make up reductionist criteria like RECIST 1.1, yielding greater association with overall survival.
Top 20 biopharma sponsors apply our deep learning models to their clinical trial imaging data to unlock powerful insights. Our models instantaneously generate Imaging-based Prognostication (IPRO) scores and thousands of Spatial Imaging Biomarkers (SIBs) from each scan, which Sponsors can combine for analysis with other data modalities such as ctDNA. These insights are being explored by clinical development teams to quantify treatment effects earlier in order to design more successful clinical trials.
Our multi-disciplinary team is on a mission to help get the most effective novel treatments to patients sooner. Altis is proudly headquartered in Toronto, Canada.
To learn more, email info@altislabs.com.